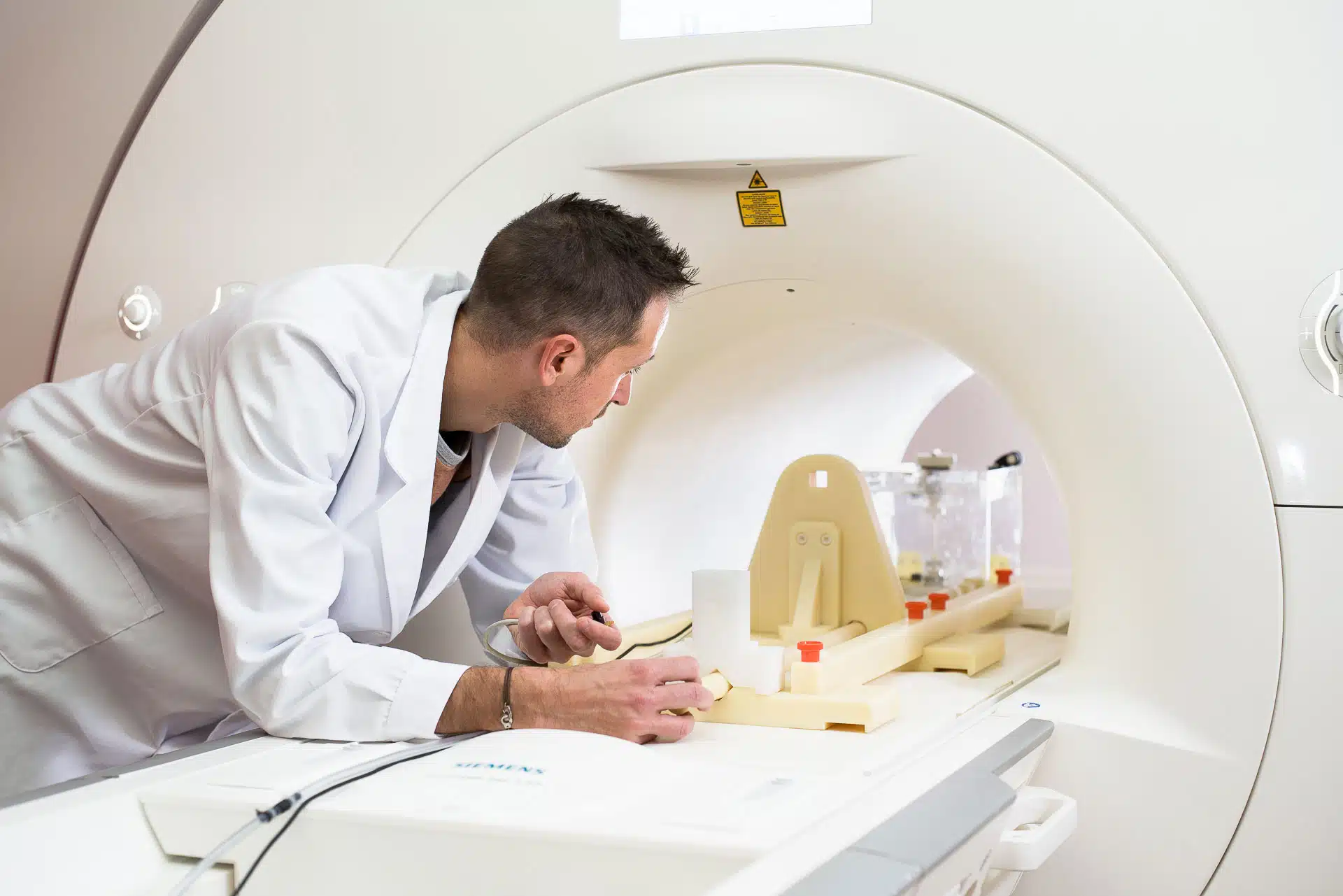
Healtis : Experts in compatibility and safety of MRI medical devices
Founded in 2012 with the combined expertise of Yannick Ponvianne, Cédric Pasquier and Jacques Felblinger, HEALTIS is a testing laboratory specializing in assessing the safety and compatibility of medical devices in the MRI environment.
Spun-off from the IADI INSERM U947 and CIC-IT INSERM CIT801 research laboratories, HEALTIS benefited from the scientific and technical expertise from their combined 20 plus years of experience in the design of instruments adapted to the MRI environment. HEALTIS was founded to respond effectively to the needs of medical device manufacturers, offering them adapted & high-quality solutions.
A Commitment to Quality at the Service of Our Customers
HEALTIS is committed to providing top-quality services thanks to rigorously selected equipment, a highly qualified and experienced team, and a proven management system based on continuous improvement.
Our services for safe medical devices in MRI
HEALTIS offers comprehensive support to medical device manufacturers to qualify and improve their safety in the MRI environment. Our services include:
- ISO 17025* accredited testing to assess the MRI safety of medical devices.
- Interpretation of test results and assistance with device marking, in accordance with ASTM F2503*.
- Numerical modeling: risk prediction and identification of worst cases.
- Technical and regulatory assistance: definition of the relevant assessment strategies and justification of worst cases.
- Support for the design of MR-compatible devices.
- Specialized training on MRI medical device safety, tailored to R&D and regulatory affairs teams.
*HEALTIS is accredited by COFRAC, according to NF EN ISO/CEI 17025 (accreditation N° 1-6320, scope available on www.cofrac.fr).
Research and Development: Innovating for MRI Safety
HEALTIS plays an active role in the advancement of the field of MRI safety, where scientific knowledge and regulatory frameworks are evolving at a rapid pace. Thanks to its innovative Research and Development programs, both in-house and in collaboration with French research laboratories, HEALTIS is helping to push the frontiers of risk assessment in the MRI environment. HEALTIS is involved with the working groups for all the relevant MRI regulatory standards and our team of experts works tirelessly to optimize existing solutions and develop new approaches adapted to the challenges faced by medical devices in the MRI environment.
Join a Team of Passionate Experts
HEALTIS is constantly on the lookout for new talent. Take a look at our opportunities and be part of innovation in MRI safety.
Scientific partners
Financial partners
This program is co-financed by the Région Grand Est, the European Regional Development Funds (ERDF) and BPI France.
Other partners
How to keep up with MRI safety news?
We regularly share news on MRI safety for medical devices, via the following channels:
- Subscribe to our linkedin page
- Follow our newsfeed
- Contact us to be notified by email of new standards and regulations.