
HomePage
Recent Posts
Read more

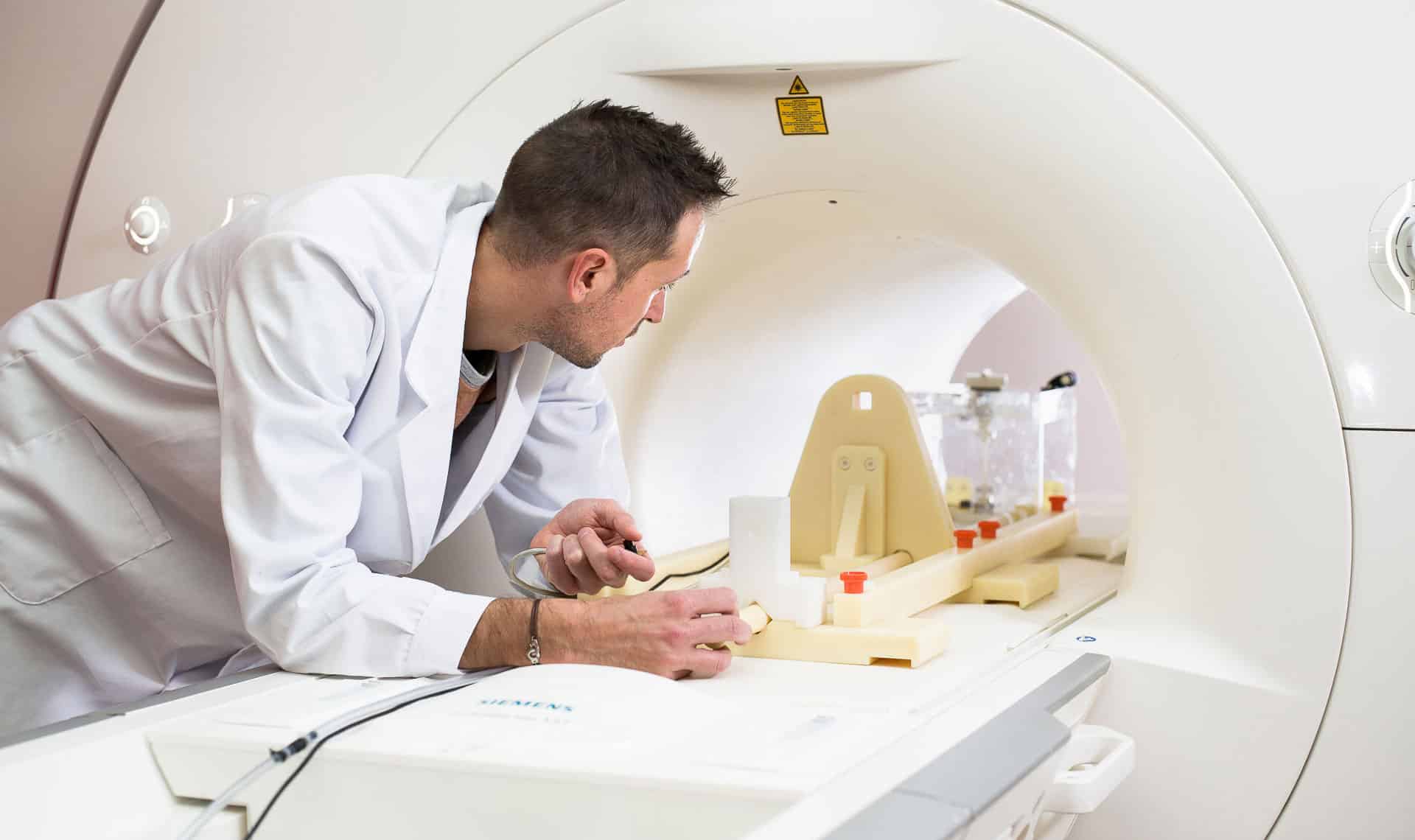
MRI safety and compatibility testing
We offer a wide range of tests for evaluating the safety of medical devices in the MRI environment +
Read more
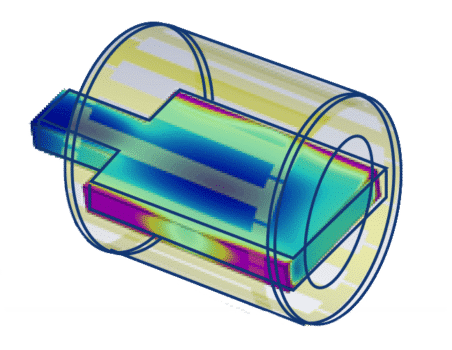
Numerical modeling
Numerical modeling allows the determination of a worstcase in a multi-feature device array. +
Read more
R&D Support
MRI involves complex and demanding physical phenomena. Designing a device compatible with MRI can be long and expensive. +
Read more
Technical and regulatory expertise
Risk assessment in MRI and development of a strategy for managing these risks. Marking aid (ASTM F2503) (...) +
Read more

Training services
Our trainings, delivered by our experts, are intended to anyone who may enter the MRI environment. +
Read more

Our commitments
HEALTIS is accredited by COFRAC, according to standard NF EN ISO / CEI 17025 for its testing activities +
Newsfeed
Read more
Check out the interview with Rada Alnnasouri, Numerical Simulations Engineer at Healtis!
Rada Alnnasouri, Numerical Simulations Engineer at Healtis, describes her journey from her school days through to her current role. 1/ Where are you from? I was born and raised in the Middle East until I obtained my e... +
Read more
MRI-induced vibration on your medical devices: assessment methods
New MRI vibration assessment method now available at Healtis Healtis enriches its test catalog by integrating the Tier 2 method for evaluating vibrations induced by MRI gradients, in accordance with ISO/TS 10974:2018 ... +
Read more
Healtis has moved!
Dear customers and partners, We are pleased to inform you that Healtis has moved into new premises within the CHRU de Nancy. More spacious, brighter, and located in immediate proximity to the MRI platform, our new fac... +
Read more
MRI Pretesting : a targeted first analysis to investigate on induced risks due to medical devices in MRI
Introduction The exposure of a medical device to the MRI environment may induce risks, especially when the device consists of conductive, magnetic or metallic materials. These risks must be assessed to meet regulatory... +
