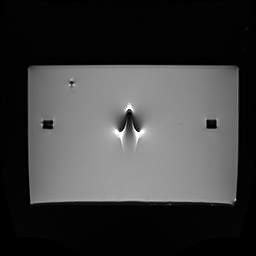
In medical imaging, alterations in images are referred to as artifacts. In MRI, the presence of a device made of ferromagnetic, paramagnetic, or conductive materials systematically leads to the formation of artifacts.
These typically appear as areas of signal void (black zones) around the device, and sometimes as image distortions. In most cases, artifacts don’t pose a major risk, but they can obscure important anatomical structures and interfere with diagnostic interpretation.
Let’s take a closer look at how these artifacts are assessed on implants.
What’s the process for evaluating MR image artifacts?
For passive implanted devices, the ASTM F2119 standard defines a method for evaluating artifacts. This standard is referenced in many others, including ISO 10974 and the FDA’s “Testing and Labeling Testing and Labeling Medical Devices for Safety in the Magnetic Resonance (MR) Environment” guide, which cite it as a possible method for assessing artifacts on active implants.
Artifacts are evaluated through testing: we acquire images with the device in place, following the protocol outlined in ASTM F2119, using MRI systems operating at 1.5 T and/or 3 T. These images are then compared to reference images taken without the device, in order to assess the extent of the artifacts caused by its presence. Artifacts are typically evaluated at each magnetic field strength (1.5T and 3T) for which MRI compatibility is claimed in the device’s labeling.
HEALTIS is ISO/IEC 17025 accredited to perform tests in accordance with the ASTM F2119 standard (Cofrac Testing Accreditation, N° 1-6320, scope available on www.cofrac.fr).
Worst-case artifact testing: how to define it?
The ASTM F2119 standard does not require artifact evaluation to be performed specifically on the configuration that generates the worst-case artifact. However, the FDA guide titled “Testing and Labeling Medical Devices for Safety in the Magnetic Resonance (MR) Environment” suggests that the largest medical device, or the one containing the greatest amount of magnetic material, can generally be considered the worst-case configuration.
How should test results be interpreted?
As noted in the FDA’s recommendations, there are generally no formal acceptance criteria for image artifacts. The purpose of this evaluation is to provide healthcare professionals with useful information in the device labeling, allowing them to assess the benefit-risk balance of performing an MRI scan on a patient with the device.
We’re here to support you
At Healtis, we guide you through every step of your study : whether it’s artifact assessment in MRI or a comprehensive assessment of all risks associated with introducing your medical device into the MRI environment.
Questions about your project? Feel free to reach out to us anytime via our contact page or at [email protected].