MRI is one of the most demanding electromagnetic environments to which a medical device can be exposed.
For medical devices made of metallic, magnetic or electrically conductive materials, exposure to MRI can generate risks that need to be assessed to gain access to commercial markets.
What about dental and maxillofacial implant systems?
Many implants, abutments, bridges, linking bars and other dental or zygomatic implant systems have been placed on the market without any risk assessment associated with their exposure to MRI, or with assessments based on rationales.
In recent years, we have noticed an alignment of requirements in accordance with those for other passive implantable medical devices. Here, we offer to clarify the topic.
What tests are to be carried out on dental implant systems?
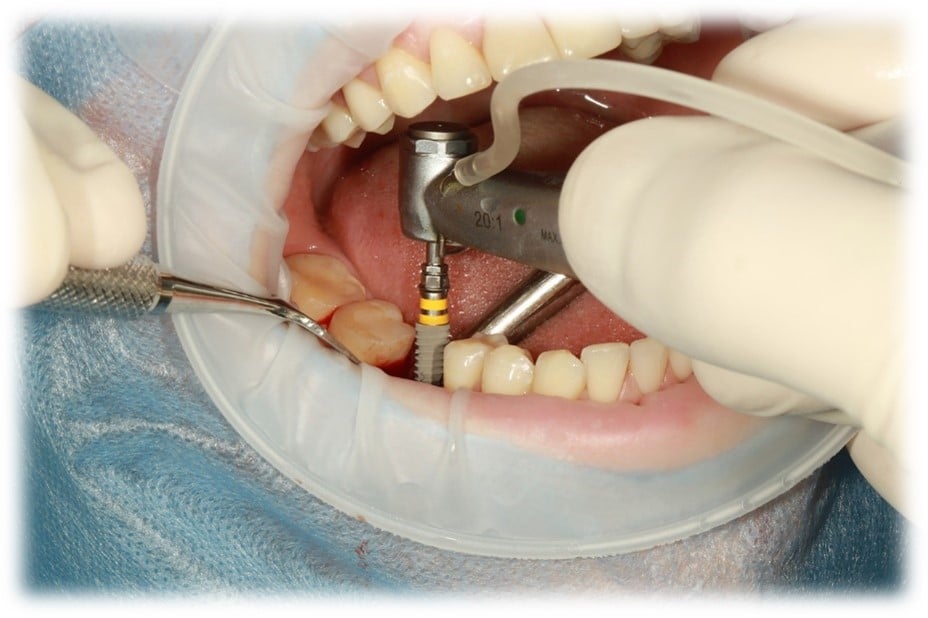
The vast majority of dental and maxillofacial systems are made of conductive materials (titanium, cobalt-chromium or stainless steel). When such a device is introduced into an MRI, several effects are induced: an attractive force and torque induced by the MRI’s static magnetic field, and heating due to the radiofrequencies present during the examination. Image disturbances (“artifacts”) are also observed in the vicinity of the implant.
These effects induce potential risks that need to be assessed in order to gain access to commercial markets. Typically, this risk assessment involves testing according to ASTM standards (F2052, F2213, F2182 and F2119).
These tests are carried out on configurations considered to be the “worst cases”. For the evaluation of force, torque and artifacts, these worst-case configurations are generally straightforward to identify, and a discussion with our team will help you in clarifying these points.
Radiofrequency induced heating is particularly influenced by device design and/or the proximity of several other devices. The variability of clinical configurations makes the worst-case identification very complex, if not impossible, without an in-depth study.
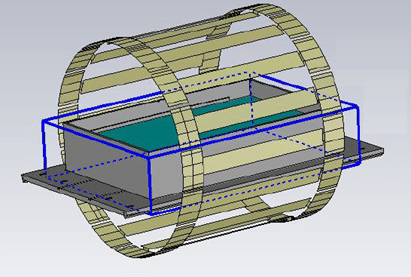
It is therefore advisable to conduct research using numerical simulations.
Focus on the use of numerical Modelling to determine the worst-case configuration on dental systems
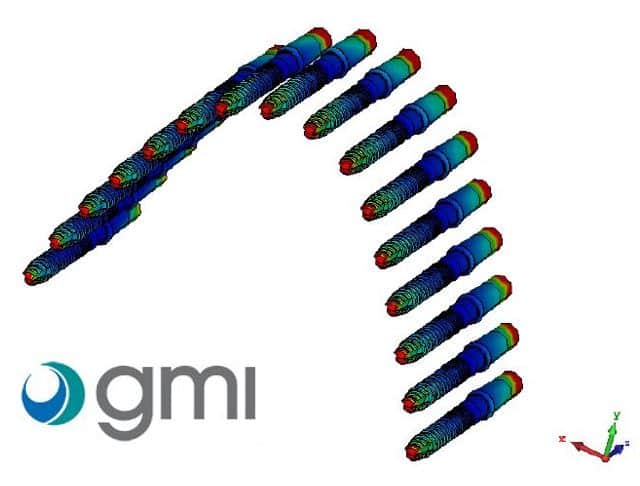
At Healtis, we offer numerical simulation services which enable us to identify a worst-case configuration: the one that will generate the most heating among the many possible clinical configurations.
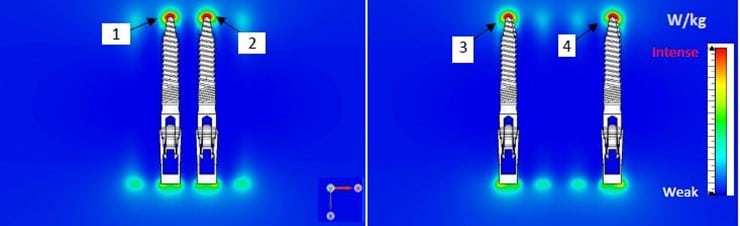
These simulations can also be used to identify hot spots on the device, which will need to be monitored during MRI testing on this worst-case configuration.
These images illustrate the Numerical simulations assessment performed by Healtis on dental implants manufactured by GMI dental Implantology, S.L.;
In 2016, the FDA published a guidance encouraging manufacturers of multi-configuration passive devices (such as dental and maxillofacial systems) to perform numerical simulations in order to identify a worst-case for the RF-induced heating assessment.
Our numerical modeling service enables us to fully respond to these recommendations, giving you the opportunity to carry out in-depth & precise studies of your medical devices.
To conclude
For dental or maxilla-facial implant systems, the main difficulty in the MRI risk assessment is generally the determination of worst-case configurations for radiofrequency-induced heating.
Simulation is proving to be an effective and accurate tool for optimizing this research. The number of configurations to be simulated will depend on the characteristics of the device and the rigor with which the manufacturer wishes to determine his worst cases. We invite you to contact us to discuss these aspects further.
Once the tests are carried out, our technical team interprets the results. This step consists of editing a report that includes the worst-case justification for each test, analyzing the results obtained and proposing a marking recommendation in line with ASTM F2503 requirements (MR safe/MR Conditional/MR Unsafe), accompanied by a paragraph on the MRI safety of your medical device to be included in your IFU.
An MRI safety study of dental and maxillofacial systems remains complex due to the diversity of clinical configurations and the proximity, or even contact, between devices in the patient’s mouth/jaw.
Any questions about the MRI compatibility of your medical devices? Reach out now at sales@healtis.com!