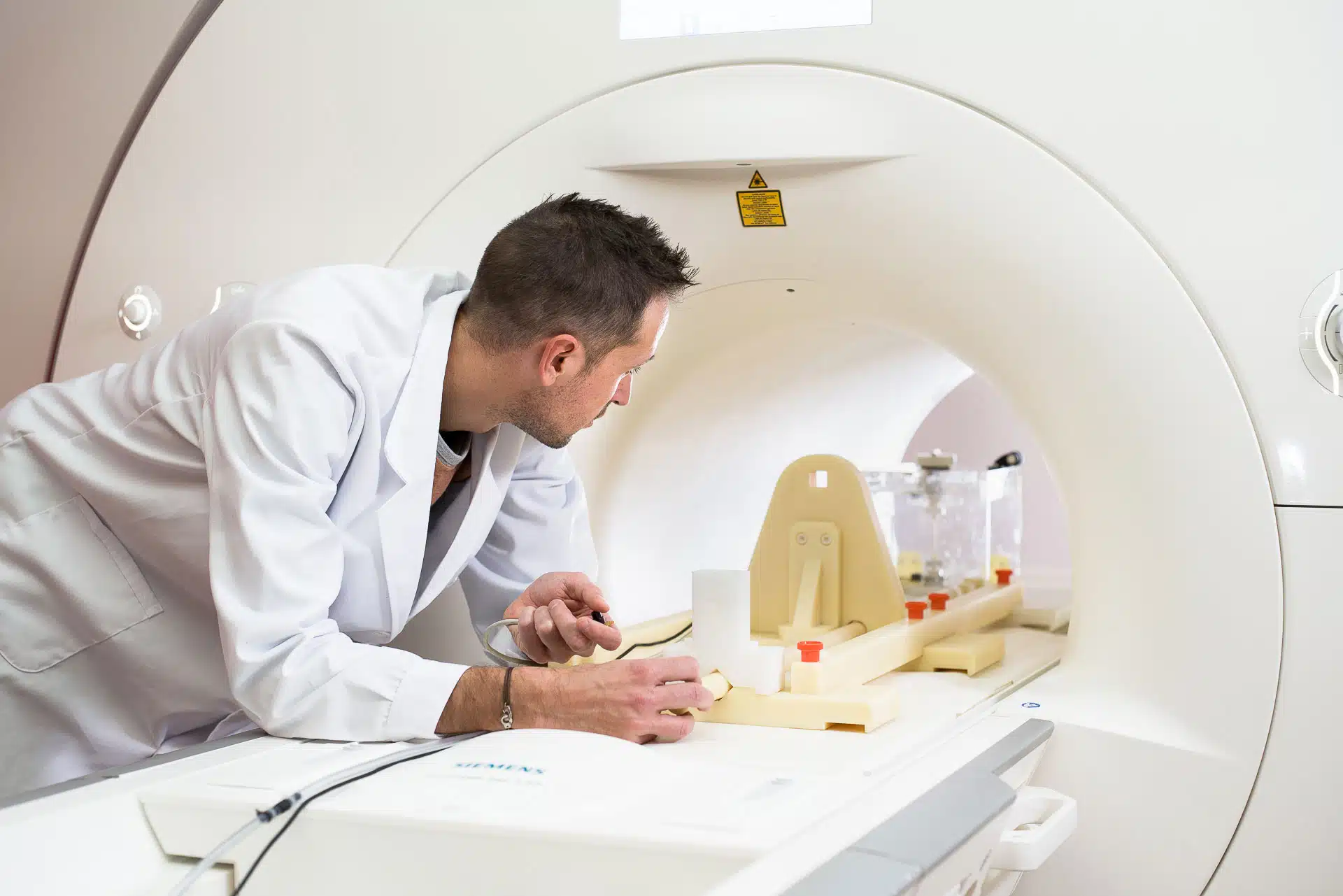
Updated in 2021, the FDA guidance document “Testing and Labeling Medical Devices for Safety in the Magnetic Resonance (MR) Environment” provides FDA recommendations for the risk assessment related to the introduction of medical devices in MRI and the suggested labelling for those devices.
Considering these risks is now necessary for regulatory submissions in the United States, as well as in most markets. We recommend reading this FDA guidance document, as it will help familiarize you with the subject.
Healtis can support you in all phases of your MRI risk assessment project. Do not hesitate to contact us at sales@healtis.com, whether you have a question about this FDA document or for any upcoming projects.